
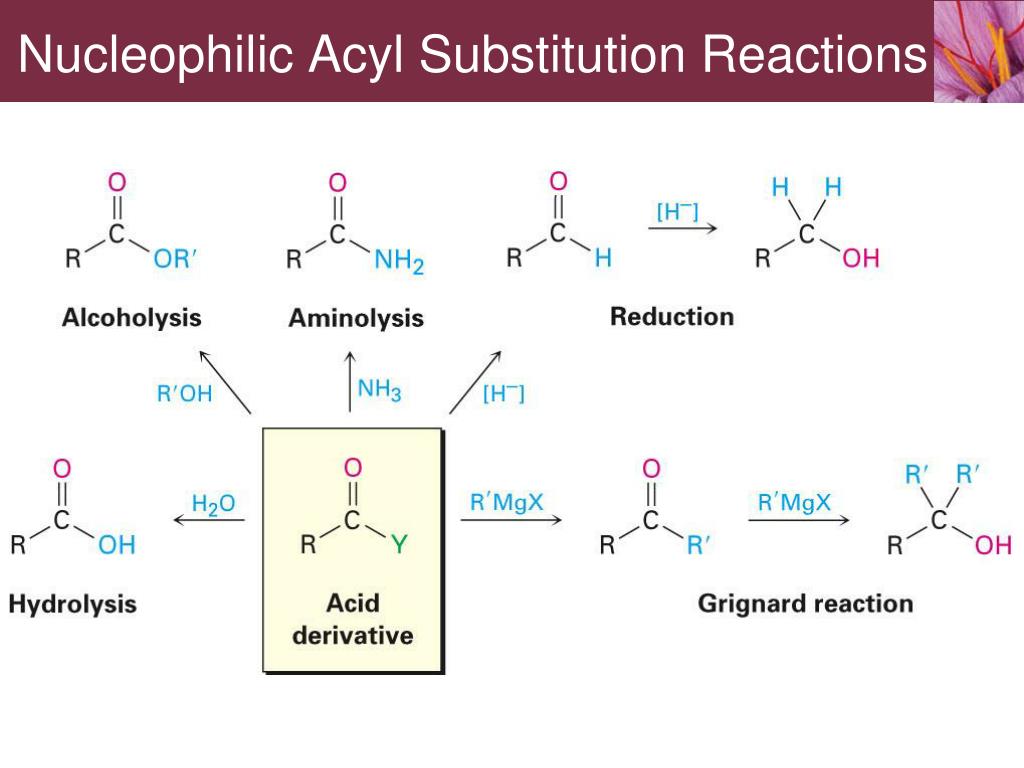
Amides do undergo acyl substitution reactions in biochemical pathways, but these reactions are inherently slow and the enzymes catalyzing them have evolved efficient strategies to lower the activation energy barrier.Ĭarboxylic acids and esters are in the middle range of reactivity, while thioesters are somewhat more reactive. Acyl Group Substitution This is probably the single most important reaction of carboxylic acid derivatives. In amides, the nitrogen atom is a powerful electron donating group by resonance – recall that the carbon-nitrogen bond in peptides has substantial double-bond character – thus amides are relatively unreactive. Carboxylic acids react with dilute aqueous sodium hydroxide solution to form the water soluble. The negatively charged oxygen on the carboxylate group has lots of electron density to donate, thus the carbonyl carbon is not very electrophilic. The carboxylate ion is a resonance stabilised carbanion. This depends on how much electron density the neighboring heteroatom on the acyl X group is able to donate: greater electron donation by the heteroatom implies lower partial positive charge on the carbonyl carbon, which in turn implies lower electrophilicity. Carboxylate anion acting as a nucleophile. Here we report the merger of metallic single-atom and photoredox catalysis, in the form of a Ni atom-supported carbon nitride material, for the CO coupling of carboxylic acids and alkyl. Carboxylate ions are negatively charged and only have two resonance structures, so they are somewhat nucleophilic and can undergo SN2 reactions. Since carboxylic acids can be easily deprotonated, they form a stable carboxylate ion. In order to understand the reactivity order. Nucleophilic Reactions of Carboxylate Anions.
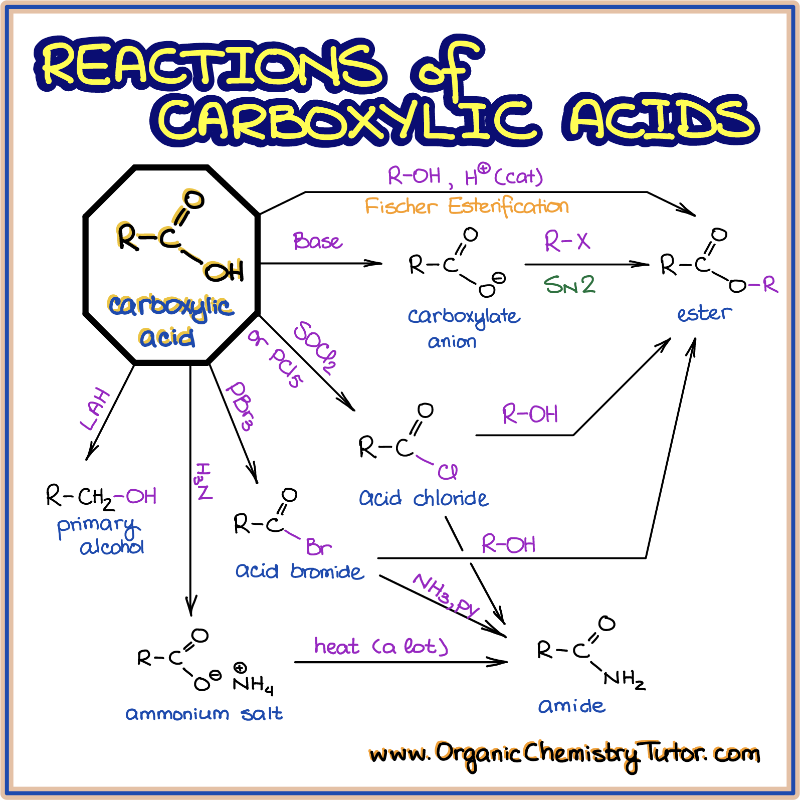
5) Understand that the reactivity of carboxylic acid derivatives depends on 2 things. In general, acid chlorides react about 1013 times faster in nucleophilic acyl substitution reactions than amides.
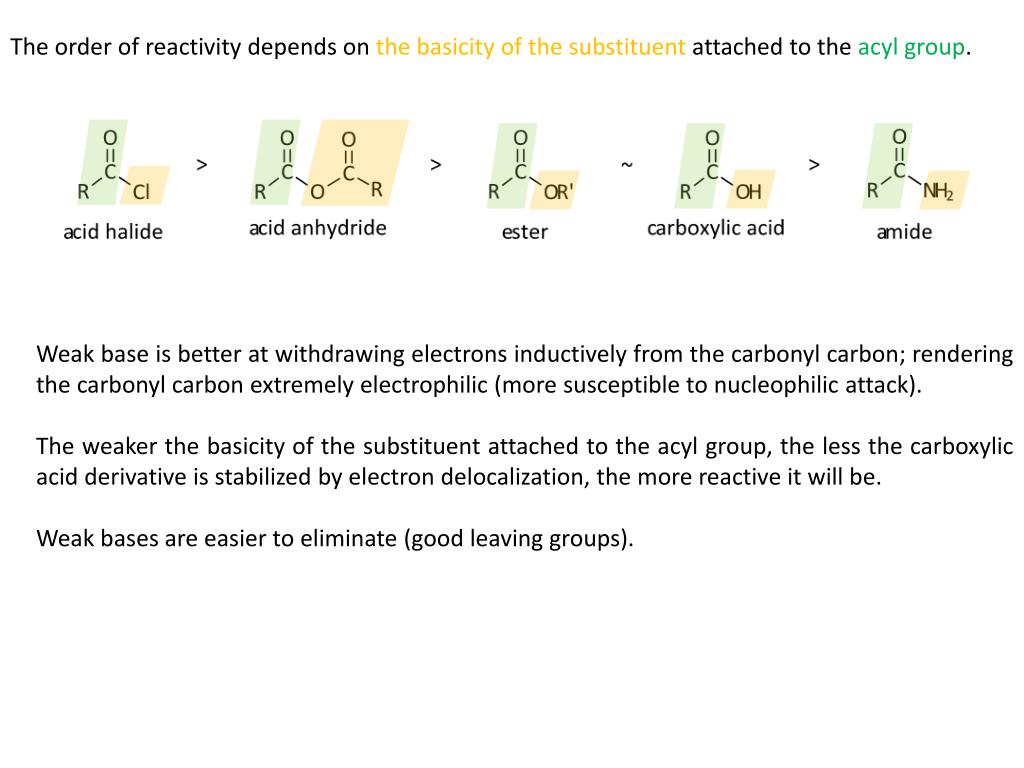
Here’s another way to think about the relative reactivites of the different carboxylic acid derivatives: consider the relative electrophilicity, or degree of partial positive charge, on the carbonyl carbon in each species. Carboxylic Acids & Carboxylic Acid Derivatives. This is why it is not possible to directly convert an ester, for example, into a thioester by an acyl substitution reaction – this would be an uphill reaction.


 0 kommentar(er)
0 kommentar(er)
